Self-dissociation of polar molecules in a confined infrared vacuum
Felipe Herrera Universidad de Santiago de Chile
Johan F. Triana (a) and Felipe Herrera (a,b)
(a) Department of Physics, Universidad de Santiago de Chile, Santiago, Chile.
(b) Millennium Institute for Research in Optics, Concepción, Chile.
Coherent light-matter interaction of molecular media in infrared (IR) cavities is a promising tool for manipulating and controlling chemical reactivity and light emission. We study the wavepacket dynamics of an individual hydrogen fluoride (HF) molecule in strongly-confined electromagnetic environments and show that in the absence of additional thermal or coherent external sources, a single-mode cavity vacuum can efficiently dissociate the molecule, when this is suddenly prepared in the vibrational ground level. We predict dissociation probabilities of up to 20% in less than 200 fs for a bare vacuum field that is resonant with the fundamental vibration frequency at the onset of the ultrastrong coupling regime. Further enhancements of the dissociation probability can be expected for a cavity with thermal excitations and multiple modes. We develop an analytical model for understanding these results using polaron transformation techniques on the multi-level quantum Rabi model of vibrational polaritons. The model highlights the importance of Bloch-Seigert shifts of the vibrational levels and the role permanent dipole moments in the light-matter coupling process. This work highlights the fundamental differences that can be expected for reactive dynamics in infrared cavities and plasmonic nanostructures in comparison with free space chemistry.
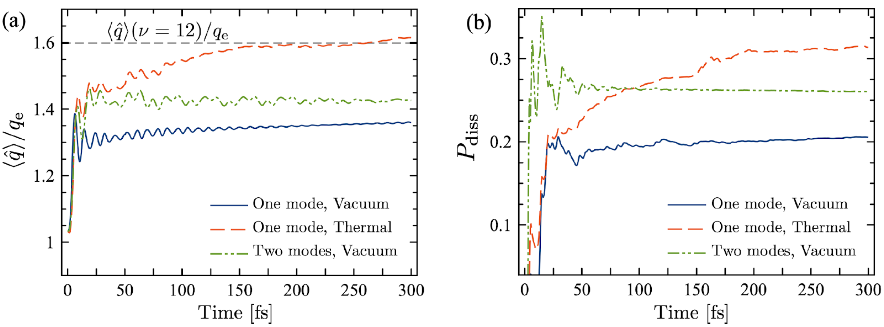
Figure 1. (a) Evolution of the mean hydrogen fluoride (HF) bond distance for different cavity states; (b) Dissociation probability for the same conditions in (a).
[1] J.F. Triana, F. Herrera, https://doi.org/10.26434/chemrxiv.12702419.v1
[2] J. Triana et al. J. Chem. Phys. 156, 124110, 2022.
[3] J.F Triana, F. Hernández, F. Herrera, J. Chem. Phys. 152, 234111, 2020.
[4] F. Hernández, F. Herrera, J. Chem. Phys. 151, 144116, 2019